Introduction
Tumor necrosis factor-alpha (TNF-α) has been the subject of intensive investigation for over thirty years 1. TNF-α is a macrophage/monocyte derived pluripotent cytokine that plays a central role in inflammation, metabolism and apoptosis 2—4. It may be regarded as one of the earliest and most critical mediators in inflammation and plays a crucial role during the early phase of a host’s defence against bacterial, viral and parasitic infections 1. In human medicine, increased levels of TNF-α have been reported in aqueous humor from human patients with AAU 5,6. Many studies have shown that in evaluating TNF-α levels, this can help to monitor disease progression and therapeutic efficacy 7—12.
In order to develop new strategies to treat ocular diseases, it is necessary to use animal models. While rodents are commonly used, ‘‘large’’ animal models (i.e. dog, cat, pig) have become increasingly attractive to assess the efficacy and safety of variety of treatment modalities that are being considered for clinical trials in human patients. The dog is a unique animal model for intraocular drug delivery studies, surgical interventions, and in vivo imaging procedures that cannot always be performed in the much smaller rodent eye. Finally, sharing the same environment as humans, the dog is affected with the same spontaneous ocular diseases 13. The dog could be a potential animal model for subsequent human trials in the field of intraocular inflammation using TNF-α as a biomarker.
To date, investigations of anterior chamber inflammation pathways in dogs were limited to aqueous humor protein contents, levels of aqueous flare and prostaglandin concentrations14—17. However, little is known about the identification of particular inflammatory mediators in the dog. Elisa kits with high sensitivity and excellent specificity for detection of canine TNF-α have only recently arrived on the market and their use is limited to research purposes. To the authors’ knowledge there is only one experimental study on the measurement of inflammatory mediators, including TNF-α, in the aqueous humor of the dog 18. There is no study from private practice measuring TNF-α levels in the aqueous humor of canine patients in a clinical setting.
The purpose of this study is to measure and to quantify the TNF-α levels in aqueous humor of normal dogs whether it is possible to obtain reference values and whether these values will be significantly lower from the values obtained in aqueous humor of dogs with intraocular pathology. We are interested in two types of frequent intraocular diseases in dogs to compare the different values of TNF-α. One group of dogs was selected with an inflammatory disease and developing in an acute fashion (AAU) and the other group with a more chronic inflammatory disease at a late stage of its evolution (primary angle-closure glaucoma).
It may seem surprising in this study to combine two very different intraocular pathologies taking into account their pathophysiology. We wanted to know if by focusing on acute disease with severe inflammation TNF-α values will be very different from those of pathology evolving on a more chronic fashion. This pilot project is used as a preliminary information-gathering exercise.
Materials and methods
Population sampling
This study was performed with the informed consent of all owners in compliance with EU animal health and welfare legislation and according to the Association for Research in Vision and the Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research.
Recruitment took place between January 2012 and July 2012. Forty-four dogs were enrolled in the study. They were all purebred dogs from 27 breeds. Thirty-one were females (13 spayed, 18 entire) and 13 males (13 entire). Twentyseven dogs (18 females and 9 males) admitted for routine neutering were used as controls. Seven animals (6 females and 1 male) presented with primary glaucoma at a chronic stage and 10 animals (7 females and 3 males) presented with AAU secondary to full thickness local puncture of the cornea by a thorn or a cat claw. No lens capsule rupture was present and the anterior chamber was never collapsed at the time of the ophthalmic examination.
All enrolled dogs were scheduled for general anaesthesia. The use of general anaesthesia was always unrelated to potential inclusion in this study. Dogs from the control group and dogs with glaucoma were included in a programme of research requiring anterior chamber oculocentesis for diagnostic purposes unrelated to this study. For the dogs with the AAU, therapy involves both medical and surgical treatment to prevent adhesions and sequelae such as pupillary seclusion. Anaesthesia was necessary to explore the corneal trauma and for the anterior chamber lavage. Canine eye responds to any insult with significantly more inflammation than human eye. All glaucomatous dogs were diagnosed with chronic primary angle closure glaucoma based on clinical signs of glaucoma (e.g. mydriasis and episcleral congestion) associated with elevated intraocular pressure (IOP), buphthalmia, abnormal gonioscopic examination of the contralateral eye, and the absence of other ocular disease. The IOP of affected eyes ranged from 30 to 65 mmHg. In accordance with the Hogan Kimura scale 19 for uveitis from 0 (absent) to 4 (severe), all uveitic dogs were scored 4.
Information recorded for each subject included breed, gender and age. All subjects underwent a general physical and ophthalmic examination prior to aqueous humor sampling. For the control group, no apparent ocular disease was present at the time of the paracentesis.
Complete blood count and serum biochemistry profiles were performed in each case before general anaesthesia.
In the control group, the ratio of albumin/globulin (A/G) was found to be between 0.8—2.2 for all dogs reflecting the absence of a generalized inflammatory state 20.
Dogs having topical or systemic anti-inflammatory or prostaglandins administered within fifteen days prior to sample collection were excluded from the study.
All dogs were examined by the same veterinary ophthalmologist and underwent screening that included the assessment of vision, photomotor reflexes, measurement of intraocular pressure (Tono-Pen® XL; Mentor Worldwide LLC Ltd), examination by slit lamp (SL-15 Portable Slit Lamp Biomicroscope; Kowa Co. Ltd), gonioscopy (Ocular Kœppe Diagnostic Gonioscopy Small Lens 17 mm; Eickemeyer Veterinary Equipment Inc.) and fundic exam (Omega 500 LED indirect ophthalmoscope; Heine Optotechnik Ltd and Volk Pan Retinal 22 diopter indirect ophthalmoscopy lens; Nidek S.A.).
All dogs were re-examined by the same ophthalmologist on days 1, 2, 7 and 15 after anterior chamber oculocentesis.
Sample collection and handling
Dogs were premedicated with an intramuscular combination of acepromazine at a dose of 0.03 mg/kg (Calmivet®; Vetoquinol, Lure, France) and morphine at a dose of 0.4 mg/kg (Morphine Aguettant®; Aguettant, Lyon, France) 30 minutes before induction. Anaesthesia was induced by intravenous injection of 2 mg/kg alfaxalone (Alfaxan®; Vetoquinol, Lure, France) to effect for intubation and maintenance with isoflurane and oxygen (Vetflurane®; Virbac, Carros, France).
A single aqueous humor sample was collected immediately after the anaesthesia for the glaucoma group and before the start of surgery for the control group. For dogs with AAU, the aqueous humor was collected just before the anterior chamber lavage at the time of surgery. Only one eye per dog was sampled.
Eyelids were cleaned with saline diluted (50:50) povidone-iodine solution (Vétédine solution®; Vetoquinol, Lure, France). The cornea was irrigated with a 1:50 saline dilution of povidone-iodine solution for 1 min, prior to rinsing with aseptic irrigation solution (Ocryl®; TVM, Lempdes, France). The dogs were then placed in lateral recumbency and an eyelid speculum was placed. Anterior chamber oculocentesis was performed using a 1-mL insulin syringe with a 27 gauge fixed needle. The needle was inserted 1—2 mm posterior to the temporal limbus and tunnelled towards the anterior chamber to minimize leakage and to avoid any bleeding. During the collection, 3—4 mm of the needle tip could be visualized in the anterior chamber and care was taken not traumatize the iris or lens.
At least 0.20 mL but no more than 0.30 mL of aqueous humor was collected per eye, sufficient for measurement while avoiding total collapse of the anterior chamber.
Following removal of the needle, the insertion site was manually compressed for 30 seconds.
The aqueous humor samples were centrifuged for 20 minutes at 1000 × g. The supernatant was removed. The samples were then sent immediately to a specialized laboratory to perform the assays (Laboratoire CAL; Troyes, France). The collected samples were frozen at —80 ◦C.
Measurement of TNF-α
The Elisa kit for tumor necrosis factor-alpha (E90133Ca) used by the diagnostic laboratory was purchased from USCN Life Science Inc. The kit is a sandwich enzyme immunoassay for the in vitro quantitative measurement of TNF-α in canine serum, plasma and other biological fluids.
TNF-α concentrations were measured with an enzymeliked immunosorbent assay (Elisa) according to the manufacturer’s protocols. Each sample was measured in triplicate.
Briefly, the microtitre plate provided in the kit is precoated with a monoclonal antibody specific to canine TNF-α. One hundred microlitres of standard or sample solution are then added to the appropriate microtitre plate wells with a biotin-conjugated polyclonal antibody preparation specific for TNF-α. Next, avidin conjugated to horseradish peroxidase (HRP) is added to each microplate well and incubated. Then, a TMB substrate solution is added to each well. Only those wells containing TNF-α, biotinconjugated antibody and enzyme-conjugated avidin will exhibit a colour change. The enzyme-substrate reaction is stopped by the addition of a sulphuric acid solution and the colour change is measured with a spectrophotometer at a wavelength of 450 nm ± 10 nm. The concentration of TNF-α in the samples is then determined by comparing the optical density of the samples to the standard curve.
This assay has high sensitivity and excellent specificity for detection of canine TNF-α.
No significant cross-reactivity or interference between canine TNF-α and analogues was reported.
Statistical analysis
The statistical analysis was performed using Systat 11. The descriptive statistical analyses were expressed as mean, median and interquartile range (IQR). The assumption of normality for each group was tested by a Shappiro-Wilk test. Since the data was not normal, a Kruskal—Wallis test was used to evaluate the effect of the independent categorical variables (sample type and disease group) on the dependent variable (tumor necrosis factor concentration). Post-hoc tests were run to compare each subgroup. A second analysis was performed using a Kruskal—Wallis test to compare the TNF-α concentration for dogs with uveitis and dogs with primary glaucoma. Final statistical significance was set at P < 0.05.
Results
TNF-α levels (Table 1) were significantly higher (P = 0.001) in the aqueous humor of dogs from the ocular disease group (mean = 308.7; median = 56; IQR = 507.5) compared to the control group (mean = 1.1; median = 0.5; IQR = 1) (Fig. 1). TNF-α levels were significantly higher (P = 0.001) in the aqueous humor of dogs from the group with PCAG (mean = 19.4; median = 20; IQR = 33) compared to the control group (mean = 1.1; median = 0.5; IQR = 1) (Fig. 2).
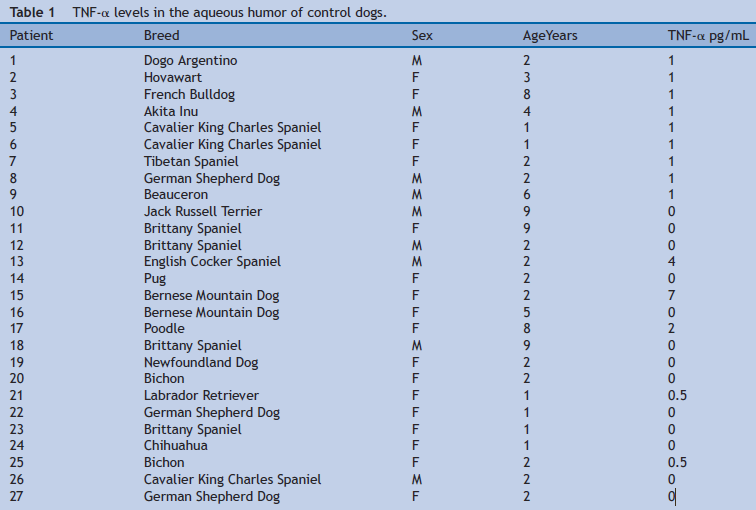
 Figure 1 Box plot diagram shows the concentration of TNF-α in the aqueous humor between the two groups.
Figure 1 Box plot diagram shows the concentration of TNF-α in the aqueous humor between the two groups.
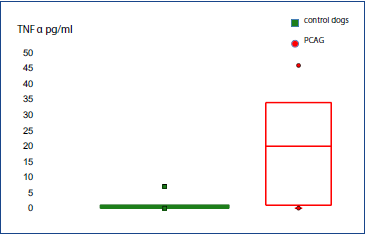 Figure 2 Box plot diagram shows the concentration of TNF-α in the aqueous humor of dogs from the control group and with primary angle closure glaucoma (PCAG).
Figure 2 Box plot diagram shows the concentration of TNF-α in the aqueous humor of dogs from the control group and with primary angle closure glaucoma (PCAG).
TNF-α levels were significantly higher (P = 0.001) in the aqueous humor of dogs from the group with AAU (mean = 511.3; median = 372; IQR = 584) compared to the control group (mean = 1.1; median = 0.5; IQR = 1) (Fig. 3).
 Figure 3 Box plot diagram shows the concentration of TNF-α in the aqueous humor of dogs from the control group and with acute anterior uveitis (AAU).
Figure 3 Box plot diagram shows the concentration of TNF-α in the aqueous humor of dogs from the control group and with acute anterior uveitis (AAU).
In the group with ocular disease, TNF-α concentrations (Table 2) were compared between the group of dogs with PCAG (mean = 19.4; median = 20; IQR = 33) and the group of dogs with AAU (mean = 511.3; median = 372; IQR = 584), TNF-α levels were higher in the aqueous humor of the uveitis group (P = 0.001) (Fig. 4).

 Figure 4 Box plot diagram shows the concentration of TNF-α in the aqueous humor of dogs with acute anterior uveitis (AAU) or primary closed-angle glaucoma (PCAG).
Figure 4 Box plot diagram shows the concentration of TNF-α in the aqueous humor of dogs with acute anterior uveitis (AAU) or primary closed-angle glaucoma (PCAG).
None of the dogs in the normal control group showed any ocular complications attributable to the anterior chamber oculocentesis on day 15 after sampling.