Introduction
Hypothyroidism is one of the most common endocrinopathy diagnosed in adult dogs.1 While it is not the purpose of this paper to review clinical signs, tests for diagnosis, the treatment with synthetic levothyroxine (LTh)2 is both straightforward and effective3 with a continuous decrease of thyroid volume.4 Because of the increase in medical care of dogs and the efficacy of the treatment, a long-term administration of LTh is often performed.
It is well known that the fundamental action of thyroid hormone (TH) is exerted on protein metabolism to regulate multiple metabolic processes.5 The TH is essential for the normal development of the central nervous system (CNS). 6,7 Along with other components of the developing CNS the neural retina is a known target of TH signaling. 8 Within the retina, photoreceptor development is particularly sensitive to manipulations of TH signaling in many species such as fish 9 and other vertebrates. 10,11 Recently, many studies have demonstrated the implication of TH in cone differentiation 12 during the retinal development, growth and regeneration. 13,14 Few findings are documented in adult dogs that have been receiving LTh treatment for many months and sometimes many years.
Relatively little attention has been given to the effect of TH on the mature nervous and sensory systems. The electroretinogram (ERG), a commonly used technique for evaluating the photopic and scotopic retinal function, has been used for many years in veterinary ophthalmology. 15,16
The purpose of this study was to evaluate, with the ERG, if the retinal function of dogs affected with hypothyroidism and treated with daily oral administration of LTh is altered.
Materials and methods
Animals
The following inclusion criteria were used:
- hypothyroidism diagnosed for at least 2 years based on positive biological and clinical signs (i.e. alopecia, squamosis, hyperseborrhea, bradycardia, obesity, fatigability, and hypercholesterolemia), total T4 lower than 16 nm/L (CERI, Paris, France), basal cTSH > 0.8 ng/mL (CERI),
- complete remission of biological and clinical signs of hypothyroidism following treatment with levothyroxine (Levothyrox®, Merck-Lipha-Santé, Lyon, France; 20μg/kg per day).
Based on these criteria, three dogs were included in this study, namely, a male Bichon Maltese Bichon cross (MB;age: 10 years old; weight: 3.5 kg; cTSH at time of diagnosis: 1.5 ng/mL), a male Yorkshire Terrier (YT; age: 13 years old; weight: 3.5 kg; cTSH at time of diagnosis: 1.3 ng/mL) and a male Shetland Sheepdog (SS; age: 9 years old; weight: 15 kg; cTSH at time of diagnosis: 2.1 ng/mL).
All dogs were found to be in good physical condition following a complete clinical examination to determine if they could be safely anesthetized. A complete ophthalmological examination, including slit-lamp and indirect opththalmoscopy performed 72 h before each ERG session, did not reveal signs of an underlying retinopathy. A blood sample was performed prior to anesthesia in order to measure T4 before each ERG recording session.
Prior to anesthesia, the pupils were maximally dilated with 1% tropicamide (Mydriaticum®, Théa, France) to obtain a stable mydriasis at least 1 h before the recording session. Pupil diameter was measured before and after the ERG examination with the aid of a strabismus compass to ascertain that maximal pupillary dilation had been achieved and maintained.
The dogs were anesthetized with a single intramuscular injection of a mixture of ketamine (5 mg/kg, Imalgène®, Mérial, Lyon, France) and medetomidine (0.1 mg/kg, Domitor®, Pfizer, Orsay, France). A hot water blanket was used to maintain the body temperature of anesthetized dogs. The dogs were intubated to maintain the airway and allow for ventilation if required. The entire procedure lasted 45 min approximately.
Material
A Visiosystem (Siem Bio-Médicale, Nîmes, France) was used to generate the light stimuli (single flash and flicker) as well as record and analyze the ERG responses. Binocular ERGs were evoked with the use of two bright flashes of light (Xenon capacitive discharge adjustable photostimulators; maximum intensity: 0.81 log cd.s/m2; duration: 20μs) delivered in full field condition as previously reported.17
The eyes were maintained opened and the third eyelid prolapse avoided with the use of sterile sclero-conjunctival copper clips attached to the conjunctiva of the superior part of the globe. 18 These clips also served as the active ERG electrodes. Reference and ground electrodes were inserted subcutaneously at the level of the outer canthus and neck, respectively (sterile acupuncture needles of 0.2 mm in diameter manufactured by Asiamed, Paris, France). Corneal hydration was maintained throughout the entire procedure with the use of a carbopol gel (Ocrygel®, TVM, Paris, France). The ERG responses were amplified 10 000 times within a 0.1–300 Hz recording bandwidth (6 db per octave) and recorded over a 250-m period for flash ERGs and 500 ms for flicker ERGs.
ERG procedure
The retinal signals were obtained in photopic and scotopic conditions as previously described. 17,18 Briefly, following a preadaptation period of 3 h to a photopic background (acclimatization
period), the animals were prepared as described above and the cone function was tested against a photopic (rod-desensitizing) background of 30 cd/m2 measured at the level of the dog’s eyes (Digital Lux tester YF-1065, Paris, France). The photopic (cone-mediated) responses were evaluated first. Photopic single flash ERGs were evoked in response to 12 different flashes of decreasing intensities (maximum intensity: 0.81 log cd.s/m2, minimum intensity: –2.19 log cd.s/m2 ; average of 15 flashes at interstimulus intervals of 1.3 Hz). These data were then used to derived the photopic luminance-response function curve, as well as the ERG b-wave/a-wave amplitude ratio.
The Naka–Rushton equation [V=Vmax I(n)/(I(n) + K(n)] was used to calculate Vmax. 18,19 Vmax is the asymptotic value of the b-wave amplitude as a function of stimulus luminance, K is the intensity that produces a b-wave whose amplitude is half of Vmax and n is a dimensionless constant that controls the slope of the function and represents the degree of homogeneity of retinal sensitivity.
The photopic flicker ERG responses evoked to flashes of Imax in intensity (e.g. flash intensity that yields the Vmax) derived from the luminance response curve were evoked at a temporal frequency of 30 Hz delivered for 20 s.
Following the assessment of the cone-mediated function, the rod desensitizing background lights were then turned off and averages of five flashes of dim blue light [Kodak Wratten filter 98 (440 nm) intensity: –3.09 log cd.s/m2] delivered at a temporal frequency of 0.1 Hz were obtained at the onset of the dark adaptation process (t0) and, thereafter, at regular time intervals (t0, t4, t8, t16 and t32 min) during dark adaptation.
The above protocol was repeated in two different occasions: Session 1, immediately following a 3-day interruption of LTh treatment and Session 2, following 30 days without any interruption of LTh treatment. In the latter case, the ERG examination always took place 7 h after LTh intake.
Data analysis
The amplitude of the ERG a-wave was measured from the baseline to a-wave trough and that of the b-wave, from trough of the a-wave to the peak of the b-wave. Similarly, peak times were measured from flash onset to the peak of individual waves. For the photopic flicker ERG, amplitudes were measured from baseline to peak.
RESULTS
Pupil diameter remained constant throughout the entire recording session. Similarly, there were only minor changes in rectal temperature.
Individual T4 serum concentration values are presented in Table 1a. For all animals tested in Session 1, the T4 serum concentration, measured before each ERG session, were below the normal range (Table 1). Similarly, in Session 2, all animals yielded T4 values that were within the range of the usual values given by the referring laboratory (Table 1).
Photopic and scotopic ERG parameters measured for each of the three dogs are reported in Tables 2 and 3, respectively, while representative ERG responses are shown in Fig. 1. Amplitude measurements (a-wave, b-wave amplitudes, flicker, b/a ratio and log k) of Sessions 1 and 2 were not different from each other. In contrast, a- and b-wave implicit times were shorter in Session 2 compared to Session 1.
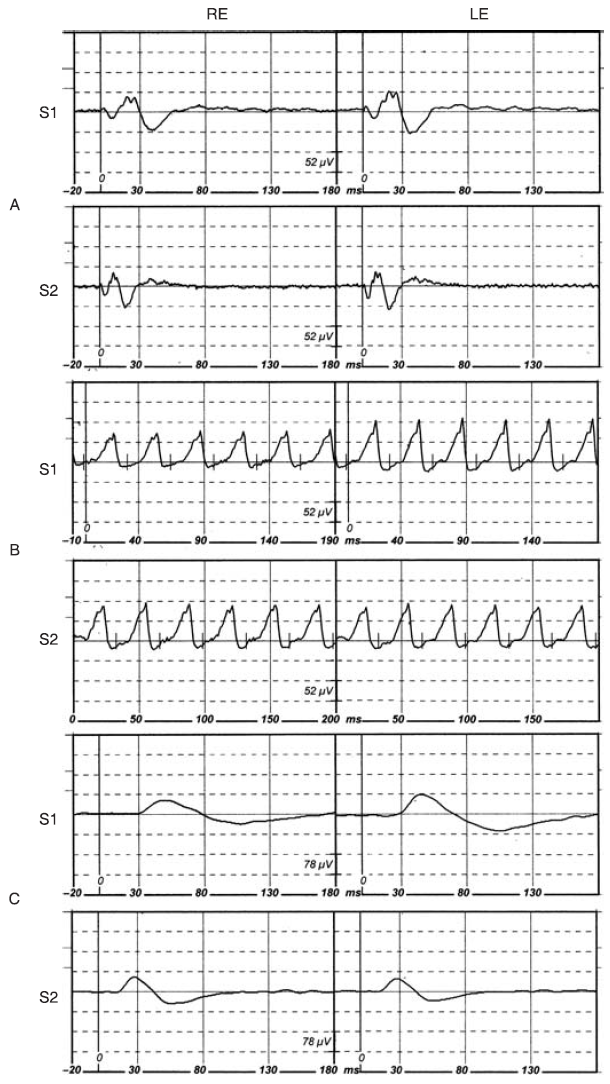
Figure 1. Representative ERGs (right eye, RE; left eye, LE) of the Shetland Sheepdog (SS)
obtained in photopic conditions: flash at Vmax (AS1 and AS2) and flicker 30 Hz (BS1 and BS2),
and in scotopic conditions at 32 min of dark adaptation (CS1 and CS2). The vertical bar (0) represents the flash onset.