Introduction
The “Evaluation of pimobendan in dogs with cardiomegaly caused by preclinical myxomatous mitral valve disease” (EPIC) study was a multicenter, blinded, randomized, placebo-controlled clinical trial valuating the effect of pimobendan in delaying the onset of clinical signs in dogs with cardiac enlargement secondary to myxomatous mitral valve disease (MMVD). The main findings and longitudinal results have been published previously.1,2
Longitudinal analysis indicated that a number of clinical, laboratory, radiographic, and echocardiographic variables changed significantly as dogs developed heart failure.1 Dogs receiving pimobendan were indistinguishable from those receiving placebo at the time of the onset of congestive heart failure (CHF).1
Previous studies have identified not only the absolute changes in clinical, radiographic, and echocardiographic variables in patients with progressive MMVD, but the time course over which these changes occur. One study documented the change in vertebral heart sum (VHS) that occurred in Cavalier King Charles Spaniels before the onset of CHF.3
The change in heart size occurred over approximately 1 year and was maximal immediately before the onset of CHF.4 Longitudinal changes in echocardiographic indicators of heart size,5 heart rate (HR), and heart rate variability6 before patients succumb to MMVD indicate a similar pattern with an increasing rate of change of the monitored variables as the disease progresses.
The increase in heart size before the onset of CHF occurs so consistently that heart size has been validated as a component of a regression equation that can be used with reasonable accuracy to predict the onset of CHF.7
Dogs developing CHF in the EPIC study showed increased heart size and HR at the time of onset of CHF compared to measurements made at baseline.1 Respiratory rate (RR), measured in the clinic, and client-measured
resting respiratory rate (RRR), measured in the dog’s home environment,
have been shown to be higher in dogs in CHF8,9 returning to more normal values once signs of heart failure are medically controlled.10
The longitudinal change in RR and RRR in a large population of dogs as they develop CHF has not been described previously. Dogs with preclinical left-sided heart disease have been shown to have a RRR that usually is <25 breaths per minute.11 Respiratory rate measured in the clinic was significantly higher at the time of onset of CHF in dogs in the EPIC study compared to results obtained at baseline. The RRR measured by owners in the home environment was not available at the baseline visit for comparison with results obtained immediately before CHF.1
As well as the previously reported changes in HR, heart size, and RR, dogs in the EPIC study that developed CHF experienced decreases in rectal temperature and body weight as they developed CHF.1
In the EPIC study, over 100 dogs were monitored as they developed CHF.2 Data from this population therefore provide a unique opportunity to determine not only the magnitude of the change in clinical and radiographic variables as CHF develops, but the time course over which those changes occur.
The aim of our current study is to describe, in a group of dogs with stage B212 MMVD, the temporal changes in clinical and radiographic variables that occur before development of CHF and to contrast the changes in dogs that developed CHF to a population of dogs known not to have developed CHF over the duration of the study.
Materials and methods
Trial design
The EPIC trial was a prospective multicenter, blinded, randomized, placebo-controlled study. Complete and detailed description of the study and longitudinal changes in measured variables have been published.1,2
The study was approved by an ethical review committee at each site where this was required.
Dogs
Enrollment criteria
Dogs were eligible for participation in the study provided the owner had given informed consent.
To be eligible for inclusion, a dog had to be ≥ 6 years of age, have a body weight (BW) ≥ 4.1 and ≤ 15 kg, have a characteristic systolic heart murmur of moderate-to-high intensity (grade ≥ 3/6) with maximal intensity over the mitral area, have echocardiographic evidence of advanced MMVD defined as characteristic valvular lesions of the mitral valve apparatus, mitral regurgitation (MR) on the color Doppler echocardiogram, and have echocardiographic and radiographic evidence of cardiomegaly defined as a left atrial-to-aortic root ratio (LA/Ao) ≥ 1.6 measured in a short-axis view,13 BW-normalized left ventricular internal diameter in diastole (LVIDDN)14 ≥ 1.7 and a VHS > 10.5.15
Exclusion criteria
Dogs were excluded from the study if they had any of the following: known clinically important systemic or other organ-related disease
that was expected to limit the dog’s life expectancy or required chronic administration of cardiovascular medication precluded as part of the trial. Dogs with hypothyroidism could be included provided the investigator deemed them clinically stable on treatment. Dogs with current or previous evidence of cardiogenic pulmonary edema, pulmonary venous congestion or both, cardiac disease other than MMVD, clinically significant supraventricular, ventricular tachyarrhythmias or both (ie, requiring antiarrhythmic treatment), or evidence of pulmonary hypertension considered to be clinically relevant (right ventricular: right atrial pressure gradient >65 mmHg) were excluded. Dogs with a history of chronic or recent administration (>14 days of duration or within 30 days of intended enrollment) of any precluded medication were excluded. In the event that, before study enrollment, a dog had received short-term treatment (<14 days) with a precluded agent, but was no longer receiving treatment and had not received it within 30 days of intended enrollment, then the dog was eligible for inclusion. Dogs that were pregnant or lactating were not eligible for enrollment.
Details of study sites, randomization and blinding, trial medication, concomitant treatment, and data management have been described previously.2
Schedule of events
Before inclusion, a case history was taken for each dog. At a baseline visit, dogs underwent physical examination, echocardiography, thoracic radiography, and routine hematology and blood biochemistry. Reexaminations were scheduled at 35 days after the baseline visit, approximately 4 months after baseline and every 4 months thereafter. Details of examinations that were undertaken on each visit are provided in Table 1.

Table 1 – Schedule of procedures undergone by animals remaining in the per-protocol population of the study at different examinations
aFour months from baseline visit and every 8 months thereafter.
bEight months from baseline visit and every 8 months thereafter.
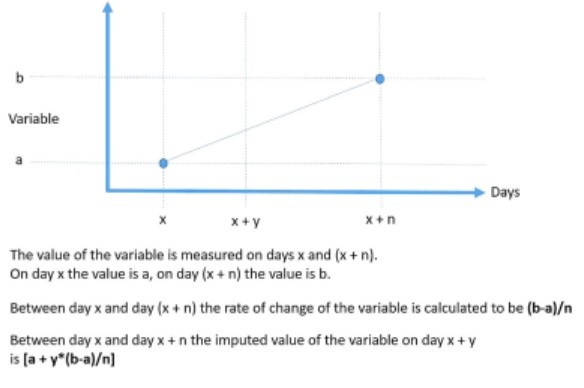
Figure 1 – The method of calculation of imputed values of continuous variables and the rate of change of variables
Clinical evaluation
At inclusion, dog characteristics such as breed, age, sex, and neuter status were noted. The BW, HR, RR, and rectal temperature (RT) were measured at each visit.
Thoracic radiography
Thoracic radiography was performed at the baseline visit, 8 months after baseline and every 8 months thereafter for as long as the dog remained in the per-protocol population. It also was performed at the time a dog was considered to have developed signs of CHF. Right lateral and dorsoventral projections were used to evaluate the thorax.
Cardiac size was assessed by the VHS method,15 and pulmonary edema and congestion were recorded, when considered to be present, by the attending cardiologist.
Resting respiratory rate
Owners also were asked to measure RRR before every reexamination.
Instruction was provided to owners who were advised to count the respiratory rate over 1 minute, in the dog’s home environment, within the week before each reexamination. Ideally, determination was performed on several days and the average of the measurements obtained was recorded as a single value at the corresponding visit.
Primary endpoint
The primary endpoint was a composite of the development of leftsided CHF verified by an endpoint committee,2 euthanasia for a cardiac reason, or death presumed to be cardiac in origin.
Statistical methods
For those dogs with verified CHF (Group CHF), the day of confirmed CHF was considered day 0. The day of measurement of different variables was expressed as the number of days before day 0. For example, if a measurement was taken 7 days before the onset of CHF, this day would be considered day −7.
For comparison, a group of dogs that were known not to have developed CHF (Group no-CHF) for at least 4 months after their final examination were included. This group consisted of dogs that were censored at the time of study closure that were known not to have experienced CHF by that time (March 1, 2015), and for which sufficient data were available to contribute to the analyses. Day 0 for noCHF dogs was defined as the day of the last visit on which all radiographic and clinical examination data were obtained (see Table 1) that was at least 4 months before closure of the study.
The values for BW were indexed to the baseline value of this variable because of the large range of values seen in the population.
Indexing was performed according to the formula 100 × (observed BW [kg])/(BW baseline [kg]). Therefore, dogs that experienced weight loss would have values less than 100.
Observations were evaluated for plausibility. All respiratory rate (RR and RRR) observations ≥100/min were assumed to represent panting and were removed from analyses.
For the 6 continuous variables recorded at least once in every 8-month period throughout the study (HR, RR, RRR, RT, BW, and VHS) for which at least 2 measurements at different time points were available for any given dog, the following were calculated: An absolute value of the variable for each dog on any day between 2 observations was calculated by interpolation for the days on which the value was not known. Absolute values of variables between visits were calculated by plotting values obtained at each visit against time for each dog, assuming a linear rate of change, and interpolating values from the plotted line to give the value of that variable for each intervening day. The rate of change of the variable was calculated for each day a dog was in the study as illustrated in Figure 1. The rate of change was assumed to be constant between 2 observations.
The daily mean of the absolute values and the daily rate of change for each group (CHF and no-CHF) were calculated for each day and plotted against time. Values for rate of change were expressed as the rate of change per month, calculated by multiplying the daily rate of change by 30. These were plotted initially by treatment group (pimobendan and placebo) and with data from dogs in both treatment groups pooled. Graphs display the curves for the 18 months (540 days) evaluated before day 0.
Finally, for the CHF group, to illustrate the temporal relationships between the different changes observed, the rates of change were expressed as a percentage of the maximum rate of change for each variable. Variables that increased with time were plotted on 1 set of axes. Variables that decreased with time were plotted on a different set of axes. No inferential statistics were performed.
Results
Dogs
Over the duration of the study, 135 dogs experienced an onset of CHF and were included in the CHF group. Of the dogs in the CHF group, 59 were receiving pimobendan and 76 placebo.
The no-CHF group consisted of 73 dogs. Of the dogs in the noCHF group, 50 were receiving pimobendan and 23 placebo. The median time in study for the CHF dogs was 414 days (interquartile range [IQR] 242-729 days). The median time in study for the no-CHF dogs was 1055 days (IQR, 976-1221 days).
The baseline characteristics of the 2 groups are summarized in Table 2. Twenty-eight from a total of 951 observations (2.9%) of RR were ≥100 breaths per minute and therefore were excluded from analyses. Twenty-five of these excluded observations were made in 18 dogs in the CHF group (4.0% of all observations in this group). In only 4 instances were these excluded observations made at the time of onset of CHF.
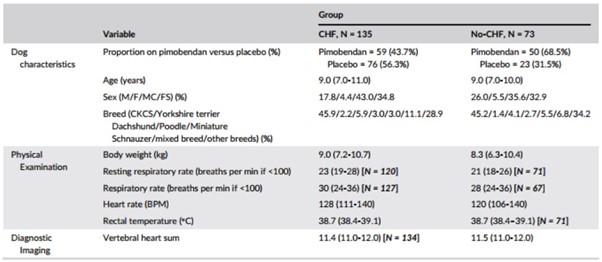
Table 2 – Baseline characteristics of the Group CHF and Group no-CHF
Note: Continuous variables are reported as median (interquartile range). Categorical variables are reported as number (%). The number of observations is reported for variables where it is different to the reported number of dogs in the group.
Three RR observations were excluded in 3 dogs in the no-CHF group (0.9% of all observations in this group). In none of these instances were the excluded observations made at the final visit. Three from a total of 859 observations of RRR were ≥100 breaths per minute and therefore were excluded from analyses.
All 3 observations were in the CHF group representing 0.6% of all observations in this group. All 3 observations corresponded to the visit at which CHF was diagnosed. One dog in the CHF group was excluded from analyses of VHS as consequence of there being a single obvious outlying data point where the magnitude of change over consecutive measurements was considered more likely to be attributable to a measurement or entry error rather than to genuine progression of disease.
Continuous variables and their rate of change
In all instances, visual inspection of plots showing absolute change and rate of change in the 6 studied variables in dogs receiving pimobendan or placebo suggested that, within the CHF group and the no-CHF group, the pimobendan and placebo groups did not behave differently.
This is illustrated for the rate of change of VHS in the CHF group in Figure 2 (similar graphs illustrating the rate of change for the CHF population in the 2 treatment groups are available in Figure S1AE). All other displayed graphs are derived from data pooling both treatment groups (pimobendan and placebo) within each group (group CHF and group no-CHF).
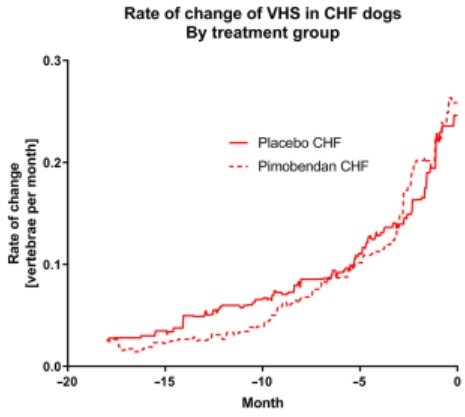
Figure 2 – The rate of change of vertebral heart sum in dogs in the CHF group separated according to the treatment they were receiving
The absolute values of the 6 studied variables for the CHF and no-CHF dogs are shown in Figure 3A-F. Body weight remained at approximately 100% of baseline for both groups until approximately 4 months before day 0. In the CHF group, mean BW decreased by approximately 3.5% in the 4 months before day 0. No similar change was seen in the non-CHF group.
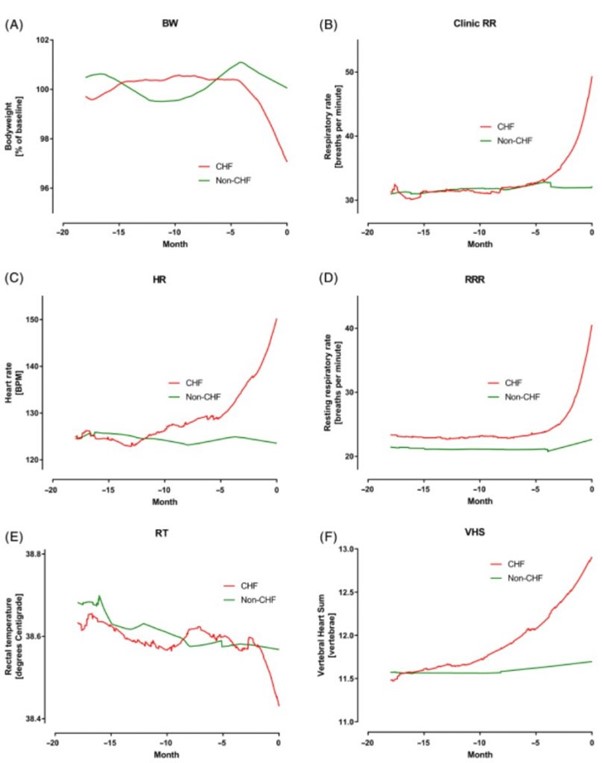
Figure 3 – A, Percent of baseline bodyweight (BW) in the 2 groups over the 18 months before day 0. B, Clinic measured respiratory rate (RR) in the two groups over the 18 months before day 0. C, Heart rate (HR) in the 2 groups over the 18 months before day 0. D, Owner measured resting respiratory rate (RRR) in the 2 groups over the 18 months before day 0. E, Rectal temperature (RT) in the 2 groups over the 18 months before day 0. F, Vertebral heart sum (VHS) in the 2 groups over the 18 months before day 0
Mean RR remained similar at approximately 31 breaths per minute in both groups until 4 months before day 0. In the CHF group, the mean RR then increased to 49 breaths per minute by day 0 while remaining approximately 30 breaths per minute in the non-CHF group. Mean RRR was in the low 20s in both groups until approximately 4 months before day 0. Over the last 4 months, mean RRR increased to approximately 40 breaths per minute in the CHF group while remaining constant in the non-CHF group.
Mean HR was approximately 125 bpm in both groups until about 10 months before day 0. Over the 10 months before day 0, it increased to approximately 150 bpm in the CHF group while remaining at approximately 125 in the non-CHF group. Mean RT was approximately 38.6°C in both groups until the final 2 months before CHF.
In the last 2 months, RT decreased by approximately 0.2°C in the CHF group while remaining 38.6°C in the non-CHF group. Finally, mean VHS initially was approximately 11.5 in both groups.
In the CHF group the mean VHS gradually increased over the 12 months before day 0 to 12.9 at the onset of CHF. In the non-CHF group, VHS did not change over the 18-month period.
The rate of change per month of the 6 studied variables is shown in Figure 4A-F. The temporal relationship of the maximum change of the 4 studied variables in the CHF dogs that increased is shown in Figure 5. The temporal relationship of the maximum change of the 2 variables in the CHF dogs that decreased is shown in Figure 6. For all variables, the maximal rate of change was observed in the period immediately before the onset of CHF.
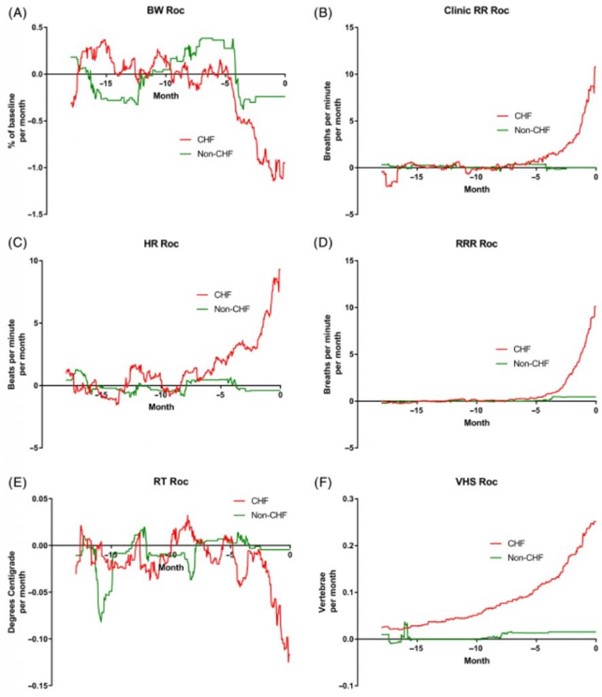
Figure 4 – A, Rate of change (Roc) of bodyweight (BW) in the 2 groups over the 18 months before day 0. B, Roc of clinic measured respiratory rate (RR) in the 2 groups over the 18 months before day 0. C, Roc of heart rate (HR) in the 2 groups over the 18 months before day 0. D, Roc of owner measured resting respiratory rate (RRR) in the 2 groups over the 18 months before day 0. E, Roc of rectal temperature (RT) in the 2 groups over the 18 months before day 0. F, Roc of vertebral heart sum (VHS) in the 2 groups over the 18 months before day 0
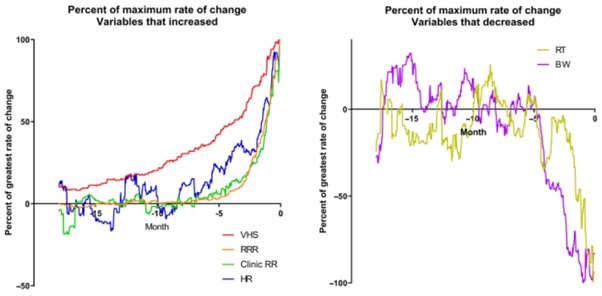
Figure 5 – Percent of the maximum rate of change for the 4 variables that increased over time expressed against time in the 18 months up to and including day 0
Figure 6 – Percent of the maximum rate of change for the 2 variables that decreased over time expressed against time in the 18 months up to and including day 0