Materials and Methods
Trial Design
The EPIC trial was a prospective multicenter, blinded, randomized, placebo-controlled study. Complete and detailed description of the study has been published.1
Dogs
Enrollment Criteria
Dogs were eligible for participation in the study provided that the owner had given informed consent.
To be eligible for inclusion, a dog had to be 6 years of age or older, have a body weight ≥ 4.1 and ≤ 15 kg, have a characteristic systolic heart murmur of moderate-to-high intensity ( ≥ grade 3/6) with maximal intensity over the mitral area, have echocardiographic evidence of advanced MMVD defined as characteristic valvular lesions of the mitral valve apparatus, mitral regurgitation (MR) on the color Doppler echocardiogram, and have echocardiographic and radiographic evidence of cardiomegaly defined as a left atrial-to-aortic root ratio (LA/Ao) ≥ 1.6 measured in a shortaxis view,14 body weight normalized left ventricular internal diameter in diastole (LVIDDN)15 ≥ 1.7, and a vertebral heart sum (VHS) >10.5.16
Exclusion Criteria
Dogs were excluded from the study if they had any of the following: known clinically important systemic or other organ-related disease that was expected to limit the dog’s life expectancy or required chronic administration of cardiovascular medication precluded as part of the trial. Dogs with hypothyroidism could be included provided the investigator deemed them clinically stable on treatment. Dogs with current or previous evidence of cardiogenic pulmonary edema, pulmonary venous congestion or both, cardiac disease other than MMVD, clinically significant supraventricular, ventricular tachyarrhythmias or both (ie, requiring antiarrhythmic treatment), or evidence of pulmonary hypertension considered to be clinically relevant (RV:RA pressure gradient >65 mmHg) were excluded. Dogs with a history of chronic or recent administration (>14 days of duration or within 30 days of intended enrollment) of any precluded medication were excluded.
Dogs that were pregnant or lactating were not eligible for enrollment. In the event that before study enrollment, if a dog had received short-term treatment (<14 days) with a precluded agent, but was no longer receiving treatment and had not received it within 30 days of intended enrollment, then the dog was eligible for inclusion.
Details of study sites, randomization and blinding, concomitant treatment, and data management have previously been described.1
Trial Medication
Pimobendan veruma was administered PO at a target dose of 0.4–0.6 mg/kg/d as per registered label instructions in most countries where the product was licensed. Placebo was administered in a similar manner. Placebo and verum tablets and packaging containers were visually identical.
Schedule of Events
Before inclusion, a case history was taken for each dog. The dog then underwent a physical examination, echocardiography, thoracic radiography, and routine hematology and blood biochemistry (performed at laboratories local to each investigator) with a minimum database consisting of packed cell volume (PCV), alanine aminotransferase (ALT), glutamic pyruvate transaminase (GPT), total protein concentration (TPC), creatinine, potassium (K+), and sodium (Na+) concentrations. Re-examinations were scheduled at day 35 and approximately 4 months after inclusion.
Thereafter, the dogs were scheduled for re-examination every 4 months. Details of examinations that were undertaken on each visit are provided in Table 1.
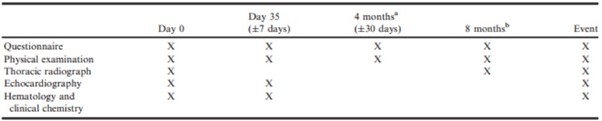
Table 1. Schedule of procedures undergone by animals remaining in the per-protocol population of the study at different examinations.
a4 months from day 0 and every 8 months thereafter.
b8 months from day 0 and every 8 months thereafter.
Clinical Evaluation
At inclusion, dog characteristics such as breed, age, sex, and neutering status were noted. The body weight, body condition score (BCS), rectal temperature, and respiratory rate were measured at each visit.
Echocardiography
Echocardiography was performed on unsedated dogs. The following measurements were each taken over at least 3 cardiac cycles, and the mean was recorded as follows: the LA/Ao obtained from the right parasternal short-axis 2D view as previously described,14 the left ventricular internal diameter at end-diastole (LVIDd), and left ventricular internal diameter at end-systole (LVIDs) measured on the M-mode echocardiogram, obtained from the right parasternal short-axis view.17 M-mode values were used to derive the fractional shortening (FS%). Normalized dimensions15 were calculated according to the following formulae:
- normalized LVIDd (LVIDDN) = LVIDd(cm) / (BW (kg))0.294;
- normalized LVIDs (LVIDSN) = LVIDs(cm) / (BW(kg))0.315.
Thoracic Radiography
Thoracic radiography was performed at inclusion, 8 months after inclusion and every 8 months thereafter for as long as the dog remained in the per-protocol (PP) population. It was also performed at the time a dog was considered to have developed signs of CHF. Right lateral and dorsoventral projections were used to evaluate the thorax. Cardiac size was assessed by the VHS method,9 and pulmonary edema and congestion were recorded, when considered to be present, by the attending cardiologist.
Primary Endpoint
The primary endpoint was a composite of the development of left-sided CHF verified by an endpoint committee, euthanasia for a cardiac reason, or death presumed to be cardiac in origin. The primary outcome variable of the study was the time period from inclusion until the primary endpoint was reached.
Visit data for the day 35 visit were included in the analyses provided the dog was re-examined between 28 and 42 days (day 35 ± 7 days) after initiation of treatment. Visit data for subsequent visits were included in between-group comparisons for each visit providing the visit took place within 30 days of the scheduled date of re-examination.
Quality of life variables were assessed at baseline by an ordinal scoring system (Table S1). After baseline assessment of quality of life variables, at each subsequent visit, owners were asked to score the following characteristics on a 5-point scale (Table 2): appetite, demeanor, exercise tolerance, fainting, respiratory effort, cough, and nocturnal dyspnea/coughing.
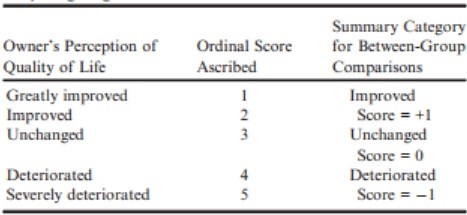
Table 2 – Ordinal scoring system used for quality of life observations; appetite, demeanor, exercise tolerance, fainting, respiratory effort, cough, and nocturnal dyspnea/coughing.
Owners were also asked to measure resting respiratory rate before every re-examination, and the average resting respiratory rate during that period was noted. Instruction was provided to owners who were advised to count the respiratory rate over one minute within the week before each re-examination. Ideally, this was performed on several days, and the average of the measurements obtained was recorded as a single value at the corresponding visit.
Statistical Methods
Any dog in the study population that was confirmed to have met all inclusion criteria (and none of the exclusion criteria) was included in the PP population until one of the following occurred:
The dog reached the primary endpoint, the dog was censored from the primary endpoint analysis due to the occurrence of an event that precluded continuation in that population, or the end of the study was reached. All analyses described herein were conducted on dogs that remained in the PP population.
For continuous variables that were measured at both baseline and day 35, a “change from baseline” variable was created by subtracting the value obtained at baseline from the day 35 value.
For every dog for which any of the clinical or radiographic continuous variables (ie, body weight, rectal temperature, respiratory rate, owner-measured resting respiratory rate, heart rate, and VHS) were measured on at least one additional visit after baseline assessment (2 occasions after baseline for owner-measured respiratory rate), a curve was constructed by plotting each data point on a graph with the unit of measurement on the vertical axis and time on the horizontal axis. The area under the curve (AUC) averaged for the number of days between the first and last observation was calculated, thus giving an average value for that variable that incorporated all of the observations made and was independent of the duration of time the dog remained in the study.18 These summary values were then compared between treatment groups.
For comparison of quality of life characteristics between groups at specific visits, dogs in each group were classified on an ordinal scale to be improved (+1), unchanged (0), or deteriorated (−1) (Table 2).
For ordinal variables measured on the same scale at baseline and all subsequent visits (ie, BCS, heart murmur intensity, and heart disease class), dogs were classified as having increased (+1), remained unchanged (0), or decreased (−1).
Between-group comparisons of the change in ordinal variables were undertaken at each scheduled visit up to the visit at which fewer than 50% of dogs remained in each group.
For all continuous and ordinal variables, between-group comparisons were made by Mann-Whitney tests. For within-group paired comparisons of all ordinal and continuous variables, the Wilcoxon signed ranks test was used.
The results of multivariable Cox proportional hazards analysis analyzing the association between treatment and clinical variables measured at baseline and the time to the primary endpoint have been described in this population.1 In this longitudinal analysis, the effects of a change in echocardiographic variables on time to the primary endpoint were explored by multivariable Cox proportional hazards analysis. Separate multivariable models were constructed for each of 4 echocardiographic variables; LVIDDN, LVIDSN, LA/Ao, and FS%. In each model, the baseline value of the variable, the change from baseline at day 35, and the treatment effect were entered as explanatory variables. Time to the primary endpoint was the dependent variable. For each model, all dogs in the PP population for which paired observations were available to derive a value for the change from baseline at day 35 were entered into each analysis. Dogs leaving the PP population for reasons other than reaching the primary endpoint were right-censored at the time of leaving the population. Variables entered into each multivariable model were assessed for collinearity with other variables in the same model and to ensure that the proportional hazards and linearity assumptions were met.
The threshold of significance was defined as P < 0.05. All analyses were 2-tailed. Statistical analyses were performed with a commercially available software program.b
Results
A flow diagram outlining the number of dogs in the PP population in each group at different time points and the number of dogs reaching the primary endpoint is illustrated in Figure 1.
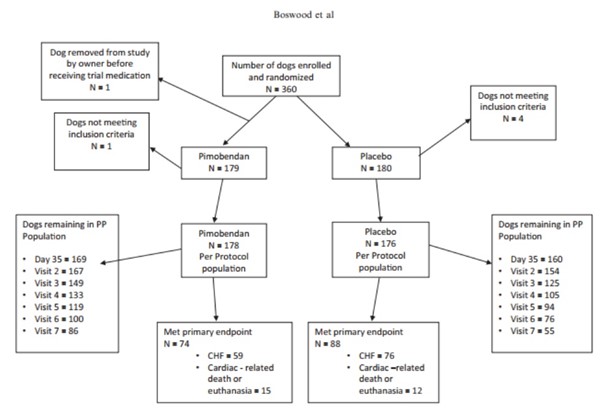
Fig. 1 – A flowchart illustrating the randomization of the 360 dogs in the study, the numbers of dogs reaching the different components of the primary endpoint in each treatment group, and the number of dogs in each treatment group remaining in the per-protocol population at different scheduled visits. PP, per protocol; CHF, congestive heart failure.
Relevant baseline characteristics of the 2 treatment groups are summarized in Table 3.
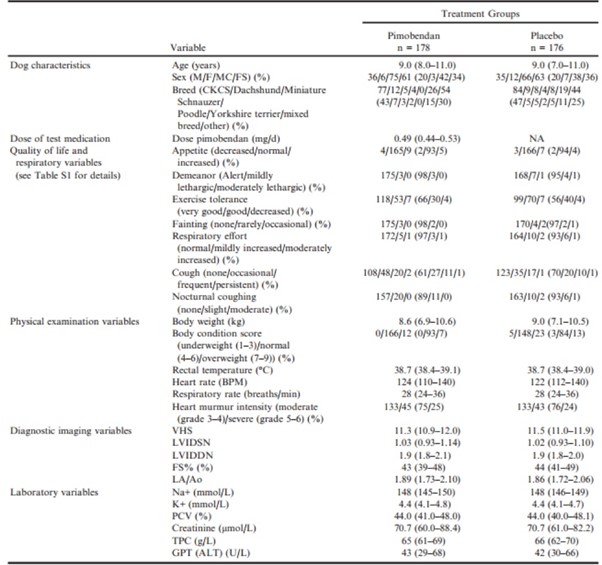
Table 3 – Baseline characteristics of the 2 treatment groups in the per-protocol population.
ALT, alanine aminotransferase; BCS, body condition score; BPM, beats per minute; CKCS, Cavalier King Charles Spaniels; F, female; FS, female neutered; FS%, fractional shortening; GPT, glutamic-pyruvate transaminase; K+, potassium concentration; LA/Ao, left atrialto-aortic root ratio; LVIDd, left ventricular internal diameter in diastole; LVIDDN, normalized left ventricular internal diameter in diastole; LVIDs, left ventricular internal diameter in systole; LVIDSN, normalized left ventricular internal diameter in systole; M, male; MN, male neutered; Na+, sodium concentration; PCV, packed cell volume; TPC, total protein concentration; VHS, vertebral heart sum.
Continuous variables are reported as median (interquartile range). Categorical variables are reported as number (%).
Comparisons at Day 35
Between-group comparisons of quality of life variables as perceived by owners at day 35 showed that the pimobendan and placebo groups were not significantly different in the extent to which any of the quality of life variables had changed (Table 4). Within-group comparisons in the pimobendan group showed the following quality of life scores to have significantly improved: appetite, demeanor, exercise tolerance, and cough (Table 4). The scores for owner perceptions of demeanor and exercise tolerance were also significantly improved in the placebo group (Table 4).
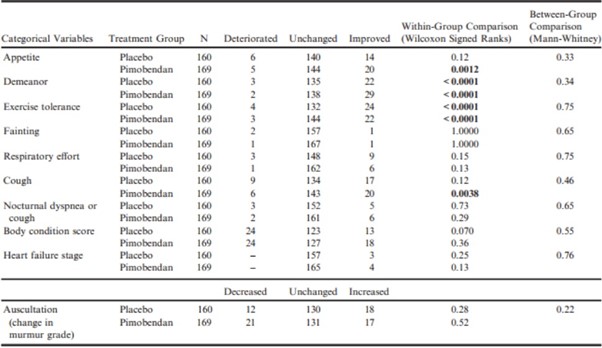
Table 4 – Within-group paired comparisons and between-group unpaired comparisons of the change from baseline to day 35 of various quality of life and physical examination variables.
All P-values that appear in bold are < 0.05.
For the physical examination, diagnostic imaging, and laboratory variables (Table 5), in the pimobendan group, there were significant decreases in LVIDDN, LA/Ao, LVIDSN, plasma Na+ concentration, and creatinine.
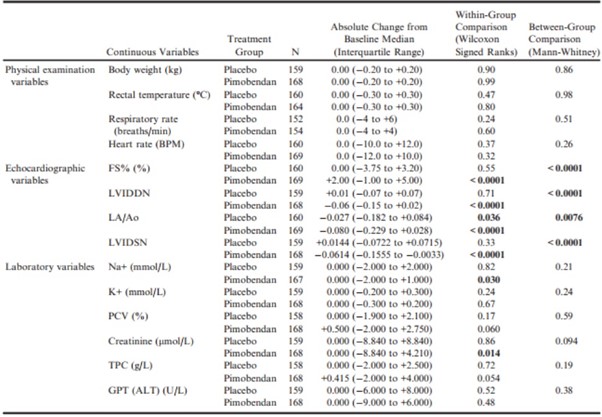
Table 5 – Within-group paired comparisons and between-group unpaired comparisons of the change from baseline to day 35 of physical examination, echocardiographic, and laboratory variables.
ALT, alanine aminotransferase; BPM, beats per minute; FS%, fractional shortening; GPT, glutamic-pyruvate transaminase; K+, potassium concentration; LA/Ao, left atrial-to-aortic root ratio; LVIDDN, normalized left ventricular internal diameter in diastole; LVIDSN, normalized left ventricular internal diameter in systole; Na+, sodium concentration; PCV, packed cell volume; TPC, total protein concentration; VHS, vertebral heart sum.
All P-values that appear in bold are < 0.05.
There was a significant increase in FS% in the pimobendan group. In the placebo group, the only significant change was a decrease in LA/Ao. Betweengroup comparisons of the changes showed a significantly greater change in FS% in the pimobendan group, and greater reductions in LVIDDN, LA/Ao, and LVIDSN in the pimobendan group (Fig 2a–d).
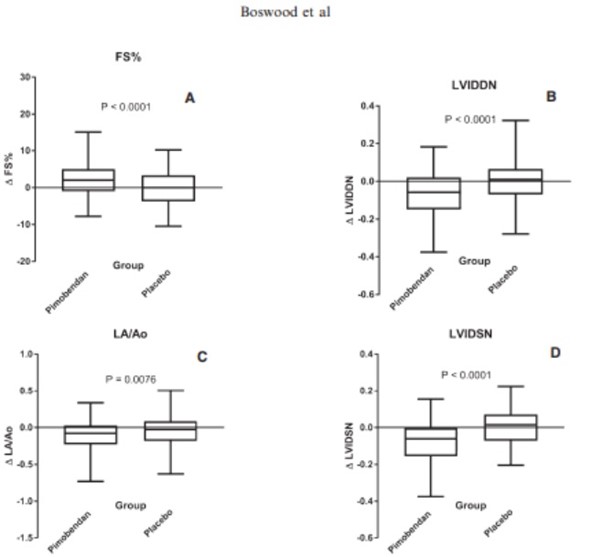
Fig. 2 – Four box and whiskers plots illustrating absolute values of the change in 4 different echocardiographic variables between baseline and day 35 in the 2 different treatment groups for (A) fractional shortening, (B) normalized left ventricular internal diameter in diastole, (C) left atrial-to-aortic ratio and (D) normalized left ventricular internal diameter in systole. The bold horizontal line indicates the median change, the boxes extend from the 25th to the 75th percentile. The whiskers extend from the 2.5th to the 97.5th percentile. P-values are for between-group comparisons by a Mann-Whitney test. Δ, change in; FS%, fractional shortening; LA/Ao, left atrial-to-aortic root ratio; LVIDDN, normalized left ventricular internal diameter in diastole; LVIDSN, normalized left ventricular internal diameter in systole.
Prognostic Value of Change in LVIDDN, LA/Ao, LVIDSN, and FS% Between Baseline and Day 35 (Multivariable Cox Proportional Hazard Analysis)
Changes in echocardiographic variables between baseline and day 35 were significantly predictive of outcome in all multivariable models, except for change in FS% (Table 6). The hazard ratio (HR) for the treatment effect remained comparably constant and significant in all the models, except for the multivariable analysis including LVIDSN and change in LVIDSN, where the observed treatment effect was apparently reduced (and no longer significant). The 4 multivariable Cox proportional hazards models are summarized in Table 6. (A Forest plot for each model is illustrated in Fig S1a–d.)
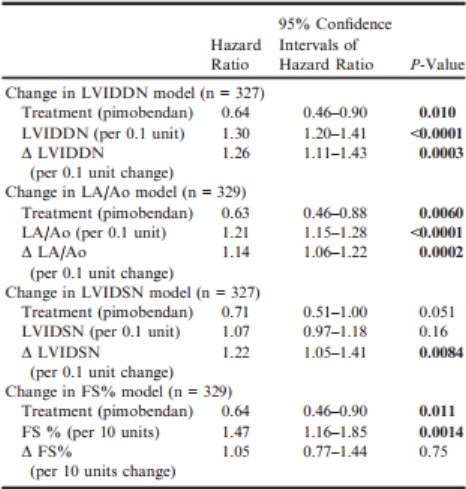
Table 6 – Multivariable Cox proportional hazards models exploring the effect of treatment, change in an echocardiographic variable, and the value of the same variable recorded at baseline on time to the primary endpoint constructed for 4 echocardiographic variables; LVIDDN, LA/Ao, LVIDSN, and FS%.
Δ, Change in; FS%, fractional shortening; LA/Ao, left atrial-toaortic root ratio; LVIDDN, normalized left ventricular internal diameter in diastole; LVIDSN, normalized left ventricular internal diameter in systole.
All P-values that appear in bold are < 0.05.
Proportional hazards and linearity assumptions were not violated, and no pair of variables entered into the same model showed unacceptable collinearity.
Effect of Treatment Over Time
The AUCs adjusted for number of days in the study for: body weight, rectal temperature, respiratory rate, owner-measured resting respiratory rate, heart rate, and VHS for each study group are summarized and compared between groups in Table 7. The median AUCs adjusted for number of days in the study for respiratory rate, heart rate, and VHS were significantly lower in the group receiving pimobendan.
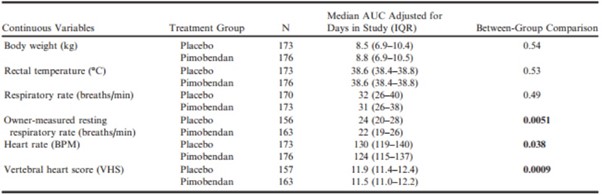
Table 7 – Comparison between groups of the areas under the curve averaged over the number of days in the study for each dog for those continuous variables that were recorded regularly throughout the monitoring period and on at least 2 occasions.
BPM, beats per minute; VHS, vertebral heart sum.
All P-values that appear in bold are < 0.05.
There were no consistent differences observed between treatment groups in quality of life scores, change in heart murmur intensity, change in heart disease stage, and change in BCS when they were compared at each visit up to the scheduled visit at 24 months, although heart failure score was worse at 20 months, and respiratory effort score was worse at 24 months in the placebo group (Table S2).
Comparison of Groups at the Onset of CHF
At the onset of CHF, there were no significant differences between treatment groups for any of the physical examination, diagnostic imaging, or laboratory variables recorded (Table 8). For all dogs that went on to develop CHF, there was, with the exception of serum Na+, K+, and PCV, a significant change from baseline to the onset of CHF in both the pimobendan and placebo group in all of the continuous clinical, diagnostic imaging, and laboratory variables recorded.
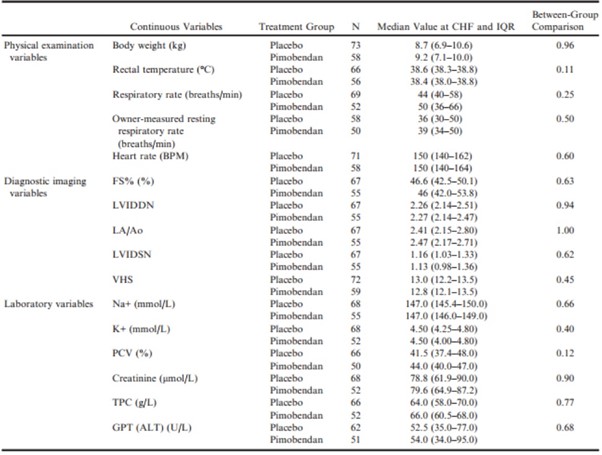
Table 8 – Values of physical examination, diagnostic imaging, and laboratory variables for those dogs that developed congestive heart failure, measured at the time of onset of heart failure summarized for the 2 treatment groups.
ALT, alanine aminotransferase; BPM, beats per minute; FS%, fractional shortening; GPT, glutamic-pyruvate transaminase; K+, potassium concentration; LA/Ao, left atrial-to-aortic root ratio; LVIDDN, normalized left ventricular internal diameter in diastole; LVIDSN, normalized left ventricular internal diameter in systole; Na+, sodium concentration; PCV, packed cell volume; TPC, total protein concentration; VHS, vertebral heart sum.
The magnitude of the change from baseline of these variables for dogs from both groups combined is summarized in Table 9.
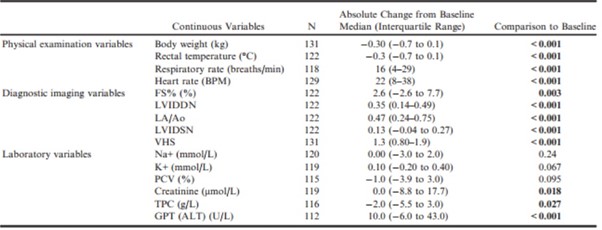
Table 9 – Change from baseline to the onset of heart failure for values of physical examination, diagnostic imaging, and laboratory variables for those dogs that developed congestive heart failure summarized for the population as a whole.
ALT, alanine aminotransferase; BPM, beats per minute; FS%, fractional shortening; GPT, glutamic-pyruvate transaminase; K+, potassium concentration; LA/Ao, left atrial-to-aortic root ratio; LVIDDN, normalized left ventricular internal diameter in diastole; LVIDSN, normalized left ventricular internal diameter in systole; Na+, sodium concentration; PCV, packed cell volume; TPC, total protein concentration; VHS, vertebral heart sum.
All P-values that appear in bold are < 0.05.
Likewise, there was a significant worsening of quality of life variables, BCS, and heart murmur intensity in their score as all dogs progressed from baseline to CHF (Table 10). Only the difference in change in exercise tolerance was significantly different between groups, with a greater number of dogs in the pimobendan group experiencing deterioration in exercise tolerance at the onset of CHF (Table 10).
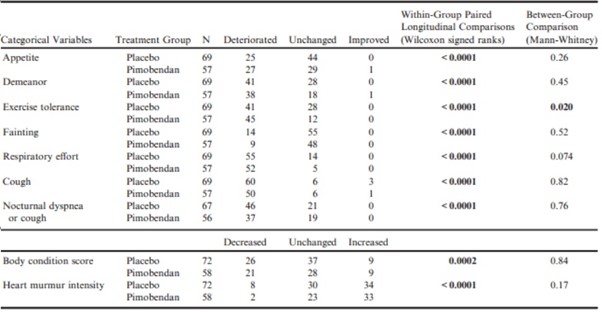
Table 10 – A summary of the number of dogs in each group that experienced a change in various clinical variables at the time of the onset of congestive heart failure.
P-values for paired comparisons with the same characteristics recorded from the same dogs at baseline are given in the penultimate column.
All P-values that appear in bold are < 0.05.