Atrial cardiomyopathy in an adult Labrador retriever dog
Abstract
A 7-year-old castrated male Labrador retriever was examined for a 10-day history of weakness and syncope.
Physical examination revealed bradycardia and a grade III/VI left apical systolic heart murmur. Electrocardiography demonstrated bradycardia, absence of P waves and an atrio-ventricular nodal escape rhythm. Echocardiography revealed marked biatrial enlargement. Thoracic radiographs showed no evidence of pulmonary edema.
Routine plasma biochemistry and electrolytes, basal serum cortisol, total thyroxin concentration, and complete blood count were within normal limits. Serum cardiac troponin I concentration was moderately increased. Serological examinations for antibodies against vector-borne diseases were negative.
A pacemaker was implanted one month after the initial presentation due to worsening of the dog’s clinical condition despite medical treatment. The dog remained asymptomatic for 18 months but was then re-presented with a gastric dilatation volvulus and subsequently euthanized.
Necropsy and histology of the heart yielded a diagnosis of atrial cardiomyopathy.
Atriale Kardiomyopathy bei einem erwachsenen Labrador Retriever
Ein 7-jähriger, kastrierter Labrador wurde für Leistungsschwäche während 10 Tagen und Synkope vorgestellt. Die Auskultation ergab eine Bradykardie und ein Herzgeräusch (Grad III/VI) über dem linken Herzapex. Das EKG zeigte eine Bradykardie, keine P Wellen und einen junktionalen (AV) Ersatz-Rhythmus. Über eine Echokardiographie konnte eine signifikante, bi-atriale Kammer-Vergrößerung dargestellt werden, aber auf dem Thorax-Röntgenbild wurde kein Lungenödem festgestellt. Blutanalysen ergaben normale Werte sowohl für Blutchemie, Serumelektrolyte, basales Serumkortisol, totales Thyroxin und Differenzialblutbild. Das kardiale Serum-Troponin war mittelmäßig erhöht. Die Antikörper-Serologie für Vektor-übertragene Krankheiten war negativ. Einen Monat nach der ersten Präsentation wurde ein Herzschrittmacher implantiert, da die klinischen
Symptome schlimmer wurden, trotz medikamentöser Behandlung. Anschließend blieb der Hund asymptomatisch, aber 18 Monate später wurde er erneut vorgestellt und wegen eines Magen Dilatation-Volvulus euthanasiert. Mittels Autopsie und histologischer Untersuchung wurde eine atriale Kardiomyopathie diagnostiziert.
Case history
A 7-year-old 35 kg, neutered male Labrador retriever dog was examined for a 10-day history of weakness and syncope (5-6 per day). On physical examination, the dog was in good clinical condition. Cardiac auscultation revealed bradycardia (heart rate: 50 beats/min) and a grade III/VI left apical systolic heart murmur. The rest of the physical examination was unremarkable.
Electrocardiography
Standard 6-lead electrocardiography (ECG) showed bradycardia, absence of P wave and a regular (ie, RR interval variation less than 10%) atrio-ventricular nodal escape rhythm at a rate of 58 beats/minute (bpm, Fig. 1).
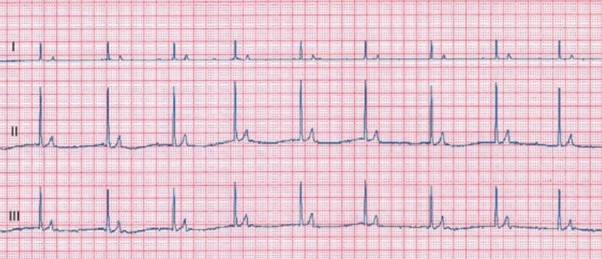
Figure 1 – Standard 6-lead electrocardiogram tracing obtained at 25 mm/s and 10 mm/mV. Note the absence of consistently definable P waves and the narrow-complex junctional escape rhythm with a rate of 58 bpm.
Echocardiography
Conventional echocardiography and standard Doppler examination were performed on the awake dog in standing position with an ultrasound unit (Megas and My Lab Twice, Esaote Biomedica, Firenze, Italy).
The left ventricular M-mode echocardiogram obtained from the right parasternal transventricular short-axis view revealed a moderate left ventricular dilation in diastole (51.0 mm) with normal systolic diameter (26.5 mm) (reference ranges (RR) of 34.0-46.3 mm and 20.5-30.8 mm, respectively, Gonçalvez et al., 2002).
The fractional shortening was moderately increased (48%, RR = 29-43%, Chetboul et al., 2005).
The two70 dimensional right parasternal transaortic short-axis view showed marked left atrial (LA) enlargement (LA to aorta ratio = 2.52, RR < 1.6, Rishniw and Erb, 2000).
The right atrium appeared dilated on the two-dimensional right parasternal long axis view with a ratio between the right and left atria of 0.8 (Fig. 2).
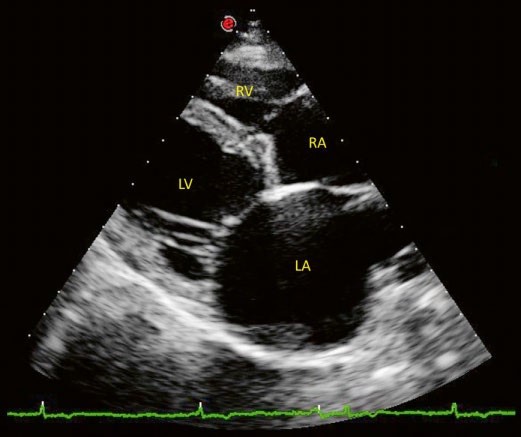
Figure 2 – Extract of the 24-hour Holter examination (leads I and II) obtained at 25 mm/s and 10 mm/mV. Note the absence of consistently definable P waves, the narrow-complex junctional escape rhythm (mean rate of 58 bpm) and the periods of ventricular escape beats.
The aspect of all cardiac valves was within normal limits except for mild thickening of the mitral leaflets.
The left apical 4-chamber view revealed moderate mitral regurgitation using Color Doppler mode (area of the regurgitation jet signal to LA area < 50%).
Finally, pulsed and continuous Doppler mode showed an increased E mitral wave velocity, reflecting LA pressure overload (2.68 m/s, RR: 0.61-1.13 m/s, Chetboul et al., 2005) and the absence of diastolic transmitral atrial phase flow.
Radiography
Thoracic radiographs revealed cardiomegaly (vertebral heart scale of 12.5, RR < 10.7, Buchanan and Bücheler, 1995) and pulmonary venous congestion but no signs of cardiogenic pulmonary edema.
Holter examination
Twenty-four-hour Holter monitoring (Fig. 3) confirmed the presence of persistent bradyarrhythmia (mean heart rate: 52 bpm) associated with ventricular escape beats at lower heart rate.
No episode of syncope was reported during the Holter examination.
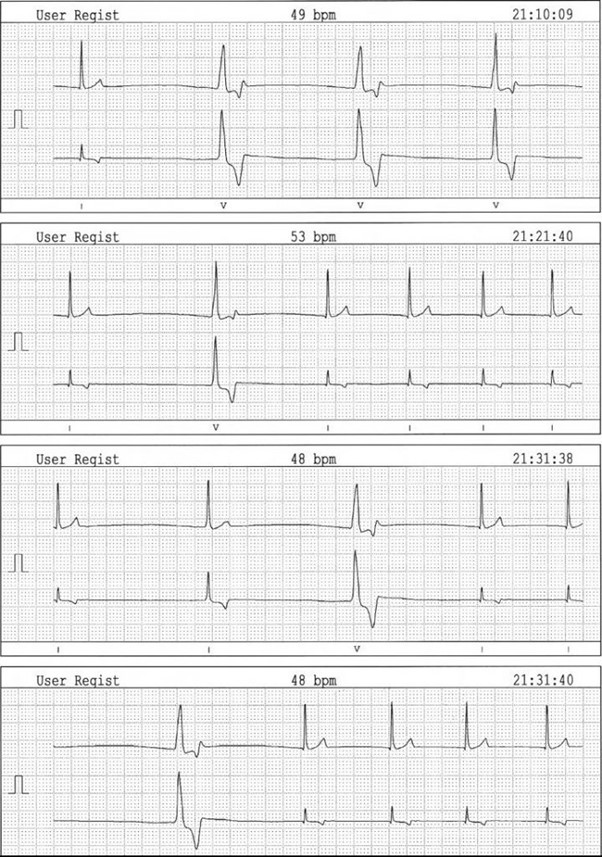
Figure 3 – Image of a two-dimensional right parasternal long axis 4-chamber view. Note the biatrial enlargement and moderate thickening of the mitral valve leaflets. LV: left ventricle, RV: right ventricle, RA: right atrium, LA: left atrium
Blood analysis
Routine plasma biochemistry and electrolytes, serum basal cortisol, total thyroxin concentrations and complete blood count were within normal limits. Serum cardiac troponin I concentration was moderately increased (0.712 ng/mL, RR < 0.06 ng/mL, Laboratoire Idexx, Alfort, France).
Finally, a qualitative ELISA test (SNAP 4Dx Test, Idexx Laboratories, Westbrook, USA) failed to detect any antibodies against vector-borne diseases such as erhlichiosis, anaplasmosis, dirofilariosis and Lyme disease.
Therapy and follow-up
The dog was medicated per os with benazepril (Fortekor, Novartis Santé Animale, Rueil Malmaison, France), 0.28 mg/kg SID, furosemide (Dimazon, Intervet, Beaucouze, France), 0.57 mg/kg BID, prednisolone (Megasolone, Merial, Lyon, France), 0.57 mg/kg BID and doxycycline (Ronaxan, Merial, Lyon, France), 8.6 mg/kg SID.
Due to the increase of syncopal episodes (ie, > 10 per day), a pacemaker implantation was scheduled one month after the initial presentation. A single chamber, permanent pacemaker (Adapta ADSR01, Medtronic, Minneapolis, USA) with VVIR (ventricular pacing, ventricular sensing, inhibition response and rate-adaptive; lower heart rate: 70 bpm; upper tracking rate: 130 bpm; amplitude: 3,5 V; pulse width : 0,64 ms) combined with a bipolar epicardial wire (CapSure Epi, Medtronics Boulogne-Billancourt, France) was implanted using a standard surgical approach (Monnet 2003).
Several left ventricular myocardial biopsies were taken during the procedure. Histopathological examination failed to reveal any degenerative, inflammatory or infectious disease of the ventricular myocardial and epicardial tissues.
Prednisolone and doxycycline were discontinued 10 days after surgery. Follow-up included clinical, ECG, Holter and echocardiographic examinations.
Despite a decrease in serum cardiac troponin I concentration (0.105 versus 0.712 ng/mL) and ventricular diameter (48.5 versus 51.0 mm) 4 weeks after pacemaker implantation, the atria remained dilated with an LA to aorta ratio of 2.19 (versus 2.52 at first examination).
The dog remained asymptomatic and in good clinical condition for 18 months but was then presented with gastric dilatation volvulus resulting in euthanasia.
Gross examination revealed the atria to be highly enlarged and discolored with thin walls. The ventricles were moderately dilated and the atrio-ventricular valve leaflets were thickened and irregular.
Histological examination of both atria revealed severe extensive replacement fibrosis and atrophic modifications of the residual myofibers that were dissociated by interstitial collagen deposition (Fig. 4). Foci of mild interstitial fibrosis were found in the ventricles.
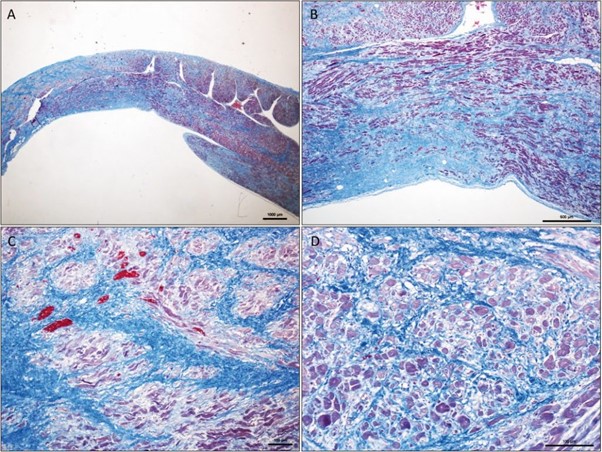
Figure 4 – Microscopic aspect of the right atrial wall at low magnification. Severe extensive interstitial fibrosis dissociating
and replacing the myocardial fibers. Residual myofibers are dissociated by interstitial collagen deposition and exhibit atrophic changes. Masson trichrome stain, original magnification ×10 (A), ×40 (B), ×100 (C), ×200 (D)